Contents
Passive or active?
Consider speciation, temporal variability, uncertainties, detection limits, stratification, and cost, when weighing passive versus active water sampling. Each method has its pros and cons.

With active sampling a well-defined water volume is isolated from the environment and quantitatively extracted. Active sampling comprises grab sampling at high or low temporal resolution, composite sampling, and continuous water sampling with autosamplers. Concentrations are calculated from extracted amounts and the water volume.
With passive sampling a sorption phase is exposed to water in situ. Transport of the target compound to the sorption phase is by diffusion and advection. Concentrations in water are calculated from the accumulated amounts and an accumulation model.
Here are some aspects to consider.
1. Speciation
Nonpolar organic compounds (for example benzo[a]pyrene) can be bound to suspended and dissolved organic matter, but the freely dissolved fraction is a more relevant proxy for bioaccumulation and toxicity at the lower trophic levels of the food web. For polar organic compounds (for example caffeine, carbamazepine) or volatile organic compounds (for example trichloroethene) speciation is not an issue, as sorption to suspended matter is minimal.
Passive and active sampling often give a different type of information
- Passive samplers target the freely dissolved fraction, because the uptake is driven by the difference in chemical activity of the target compound between sorbent and water.
- Active samplers target total concentrations. When a filtration or centrifugation step is included, a further separation can be made between concentrations in suspended matter on one hand, and concentrations of freely dissolved + colloidally bound compounds on the other.
Total concentrations can be relevant when the purpose of the monitoring study is to calculate loads that are carried by a river. Chemical activities are more relevant for risk assessment. Chemical activities can be calculated from total concentrations and vice versa, but this additional modeling comes at the price of reduced accuracy.
Key question 1:
Does the target compound bind significantly to suspended and dissolved organic matter?
- If not: both active and passive sampling can be considered.
- If so: then it depends if you want to know the total concentrations in a water sample (use active sampling) or if you are interested in the chemical activity of the compound in the water sample (use passive sampling).
2. Temporal variability
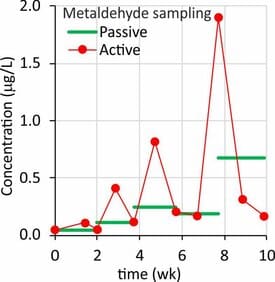
Concentrations of chemicals can vary with time, and the sampling method should yield a representative picture of compound concentrations for the time frame of interest. Nonpolar legacy contaminants often show a smooth seasonal variation that can be captured by low-frequency active sampling. Concentrations of polar agricultural chemicals and pharmaceuticals vary substantially on the time scale of days or hours (Mutzner et al., 2019; Ort et al., 2010). For these compounds low-frequency grab sampling carries a large sampling uncertainty because the sample can be taken within or between peak concentrations events. Increasing the sampling frequency can be option, but increases labor cost (high-frequency grab sampling) or equipment cost (continuous water sampling). Passive samplers are suitable for time integrated sampling over periods of days, weeks, months, depending on the sampler type and the target compound.
Key question 2:
Are concentrations of target compounds highly variable within the time frame of interest?
- If not: both low-frequency active sampling and passive sampling can be considered.
- If so: high-frequency active sampling and passive sampling can be considered.
3. Stratification
Concentrations in ground water wells may show strong vertical concentration gradients. Active sampling can distort the vertical distributions when water near the sampling inlet is withdrawn (ITRC, 2007). Gradient distortion may also occur for monitoring in the hyporheic zone of rivers (Mechelke et al., 2019). Passive samplers do not draw any water, and therefore do not induce mixing with adjacent water layers, except during deployment.
Key question 3:
Will taking a water sample distort concentration distributions?
- If not: active sampling and passive sampling can both be considered.
- If so: passive sampling is the only way to go.
4. Detection limits
To check if passive samplers meet the required method detection limits it is useful to know the water volume that is extracted after some time. At the initial stage of the sampler deployment this equivalent water volume equals sampling rate x time (Rst). As an example: atrazine sampling rate with Chemcatchers is ~0.13 L/d, and a 3-weeks exposure would extract a water volume of 2.7 L. When, for example, a minimum amount of 1 ng is needed to positively identify the occurrence of atrazine in the environment, then the detection limit would be 1/2.7=0.4 ng/L.
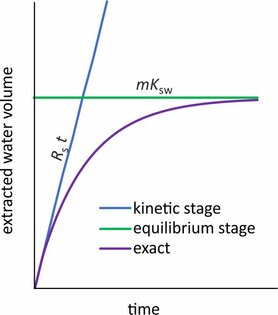
The extracted water volume is however bound to a maximum that occurs when target compounds attain sampler-water equilibrium. In this case the equivalent extracted water volume equals sorbent-mass × sorption-coefficient (mKsw). As an example: the silicone-water sorption coefficient of pyrene is ~48,000 L/kg. For a silicone strip of 3 g the maximum water volume that can be extracted equals 48,000 × 0.003 = 144 L, which is not easily achieved with active water sampling.
For an initial estimate of the extracted water volume in passive sampling it suffices to calculate Rst and mKsw , and adopt the smaller of these two values, as illustrated in the figure above.
Sorption coefficients are sometimes not available because no measurable degree of equilibrium is attained in sampler calibration studies. In this case the sorbent acts as an infinite sink, and accumulated amounts increase linearly with time. The calibration data should be inspected to verify the exposure time for which accumulation is linear, and the extracted water volume can be estimated from Rst alone (mKsw is essentially infinite).
Key
question 4:
Does passive
sampling meet the required detection limits?
- If not: consider longer passive sampler exposure times, or alternative passive sampler designs, or stick to active sampling methods.
- If so: passive sampling can be considered.
5. Passive sampler modeling
Uptake models are used to calculate compound concentrations from the amounts that are accumulated by passive samplers. The uptake kinetics of nonpolar compounds by silicone and low-density polyethylene is well understood, including the effects of temperature and flow (Lohmann, 2012; Smedes and Booij, 2012), and the model uncertainties can be obtained from error propagation. Uptake modeling of polar compounds by o-DGT also has a strong mechanistic basis (Challis et al., 2016).
Sampling rates by POCIS and Chemcatcher are less well understood (Charriau et al., 2016; Harman et al., 2012). Model uncertainties can only be derived from the consistency of reported sampling rates for these samplers (Poulier et al., 2014). There are many other passive sampler designs for which model uncertainty is hard to assess because only limited data for calibration and field testing are available.
Key
question 5:
Does the uptake
model for passive sampler of choice have a strong mechanistic basis, and are
the relevant calibration parameters consistent among different studies?
- If not: Further field validation using passive sampling with simultaneous active sampling is needed.
- If so: passive sampling can be considered.
6. Cost
Whether passive or active sampling is cheaper depends on many aspects, such as active sampling frequency and pooling strategy, risk of passive sampler theft or vandalism, speciation issues, and required accuracy. Including field validation of active versus passive sampling at one or more monitoring sites increases confidence in the data, but comes at additional cost. Aspects to include are the cost of labor, chemical analysis, and equipment, and the cost and risk of making incorrect management decisions based on unreliable data.
Key question 6:
Is cost an issue?
- If not: High-frequency active sampling is likely the best option for polar organic compounds. For nonpolar compounds it depends if total concentrations are targeted (contaminant load assessment; best done by high-frequency active sampling) or if freely dissolved concentrations are more relevant (risk assessment; best done by passive sampling).
- If so: a further cost-benefit analysis is needed to make an optimal choice between passive and active sampling.
Send your comments and questions
The above covers the main lines of choosing passive versus active water sampling. When you are short of time, PaSOC is happy to make a more detailed analysis of your specific monitoring requirements. Click How we work for further details.
At your service.
Further reading
Castle, G.D., Mills, G.A., Bakir, A., Gravell, A., Schumacher, M., Townsend, I., Jones, L., Greenwood, R., Knott, S., Fones, G.R., 2018. Calibration and field evaluation of the Chemcatcher® passive sampler for monitoring metaldehyde in surface water. Talanta 179, 57–63. https://doi.org/10.1016/j.talanta.2017.10.053
Challis, J.K., Hanson, M.L., Wong, C.S., 2016. Development and calibration of an organic-diffusive gradients in thin films aquatic passive sampler for a diverse suite of polar organic contaminants. Anal. Chem. 88, 10583–10591. https://doi.org/10.1021/acs.analchem.6b02749
Charriau, A., Lissalde, S., Poulier, G., Mazzella, N., Buzier, R., Guibaud, G., 2016. Overview of the Chemcatcher® for the passive sampling of various pollutants in aquatic environments Part A: Principles, calibration, preparation and analysis of the sampler. Talanta 148, 556–571. https://doi.org/10.1016/j.talanta.2015.06.064
Harman, C., Allan, I.J., Vermeirssen, E.L.M., 2012. Calibration and use of the polar organic chemical integrative sampler – a critical review. Environ. Toxicol. Chem. 31, 2724–2738. https://doi.org/10.1002/etc.2011
ITRC, 2007. Protocol for use of five passive samplers to sample for a variety of contaminants in groundwater (DSP-5). Interstate Technology & Regulatory Council, Washington, DC 20001. https://www.itrcweb.org/GuidanceDocuments/DSP-5.pdf
Lohmann, R., 2012. Critical review of low-density polyethylene’s partitioning and diffusion coefficients for trace organic contaminants and implications for its use as a passive sampler. Environ. Sci. Technol. 46, 606–618. https://doi.org/10.1021/es202702y
Mechelke, J., Vermeirssen, E.L.M., Hollender, J., 2019. Passive sampling of organic contaminants across the water-sediment interface of an urban stream. Water Res. 165, 114966. https://doi.org/10.1016/j.watres.2019.114966
Mutzner, L., Vermeirssen, E.L.M., Ort, C., 2019. Passive samplers in sewers and rivers with highly fluctuating micropollutant concentrations – Better than we thought. J. Hazard. Mater. 361, 312–320. https://doi.org/10.1016/j.jhazmat.2018.07.040
Ort, C., Lawrence, M.G., Reungoat, J., Mueller, J.F., 2010. Sampling for PPCPs in wastewater systems: comparison of different sampling modes and optimization strategies. Environ. Sci. Technol. 44, 6289–6296. https://doi.org/10.1021/es100778d
Poulier, G., Lissalde, S., Charriau, A., Buzier, R., Delmas, F., Gery, K., Moreira, A., Guibaud, G., Mazzella, N., 2014. Can POCIS be used in Water Framework Directive (2000/60/EC) monitoring networks? A study focusing on pesticides in a French agricultural watershed. Sci. Total Environ. 497–498, 282–292. https://doi.org/10.1016/j.scitotenv.2014.08.001
Smedes, F., Booij, K., 2012. Guidelines for passive sampling of hydrophobic contaminants in water using silicone rubber samplers. (No. ICES Techniques in Marine Environmental Sciences 52.). International Council for the Exploration of the Sea, Copenhagen. https://dx.doi.org/10.17895/ices.pub.5077